R&D in Clinical Diagnostics has been moving forward over the last quarter with several new products launching or in progress.
LGC Seracare is launching Sex Chromosome Aneuploidy (SCA) Reference Materials for prenatal genetic testing (also known as NIPT). The first reference material manufactured at the end of March is for Jacob’s syndrome, a genetic condition characterized by an extra Y chromosome (XYY) that occurs in about 1 out of 1000 boys. Discovered in the 1960s, its phenotype varies but common features include infertility, tall stature, abnormally large head, and increased distance between two body parts, typically the eyes. The material is uniquely derived from real pregnant donors, amplified, and encapsulated using LGC’s innovative technology.
LGC remains the first and sole supplier of SCA reference materials in the world, thus leading the way to advance genetic testing in reproductive health.
R&D has established a list of key technology development projects for FY23. These include advancing our liquid biopsy ctDNA technology for improved specificity, entering the epigenetics applications space with methylated DNA, engineering cells for clinical genomics applications based on technology developed by TNAC, and moving to cell free protein expression and manufacture.
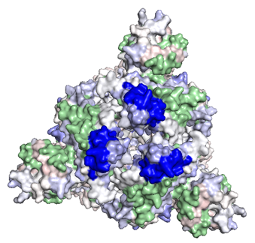
We are thrilled to report results from a multisite collaboration analyzing critical anti-SARS-CoV-2 reagents. Anti-SARS-CoV-2 humanized, monoclonal antibody mAB12459 was developed by TNAC. This antibody is a critical component and is the first VALIDATE® anti-SARS-CoV-2 quantitative serology kit. The LCMS results reported intact glycoprotein molecular weight of Mr 126,265.9 Daltons and the antigenic binding epitope on the SARS-CoV-2 spike protein amino acids 418-437. See the 3D structure below. This is a top-down view of the molecule and blue is the epitope region 418-437. The quant serology VALIDATE® product is targeted for commercial launch in late April.
This project was an outstanding collaboration. The monoclonal antibody was from Kidlington; BaseMatrix plasma diluent from Milford; formulation, product development, product management, regulatory, QA, and marketing from Cumberland Foreside; antibody quantification and antibody epitope mapping by NML Teddington. This effort is a first for our industry, as we are launching an analytically characterized antibody as a control with an unlimited supply of raw materials. Furthermore, the effort in total demonstrates LGC Clinical Diagnostics as a technology leader once again in clinical chemistry.
Yves Königshofer and Russell Garlick presented the February Keynote Invited Guest Presentation at the Frederick National Laboratory, National Cancer Institute, Frederick Maryland USA on Feb 17th. The talk, titled “Developing Standards for SARS-CoV-2 and Emerging Variants,” featured AccuPlex™ technology at the national research level for NIH sponsored research. New data from the Coronavirus Standards Working Group and new data from the LGC SeraCare Working Group on SARS-CoV-2 quantitation were presented. The presentation also included practical assay development considerations for quantitative PCR.
At Technopath, several projects are currently in progress. A Roche MS QC project is awaiting the results of a feasibility check for Vitamin D control. Development of an RMED HS Cardiac product at Technopath is slated to coincide with RMED’s new Troponin I assay. Pilots will be manufactured at Technopath and sent to Turku, Finland for analysis. Two new Multichem® releases are also in the works, and Sysmex instruments will be arriving onsite in March ahead of a Sysmex serology product development project.

.jpeg)

