Over the past quarter, the LGC CDx Commercial team has been focused on aligning commercial initiatives across the business unit with the goal of achieving “One CDx,” taking advantage of cross-selling opportunities and building a cohesive company image while continuing to generate high-quality leads.
The newly integrated Commercial team has seen three notable product launches during the past few months, attended MedLab Middle East, and hosted two customer-facing webinars.
In January, members of the Commercial team attended MedLab in Dubai under the LGC Clinical Diagnostics brand—our first outing as one unit, including Technopath Clinical Diagnostics. Sales representatives had the opportunity to speak to end users and distributors about the distinct specialties of each sub-brand. The event was well-attended and generated 94 leads, 85 of which were brand new.

Dominic Hasson, Charles Spon-Smith, Hugh Clancy, Rami El-Quaisi, and Leo McDonough representing LCG Clinical Diagnostics at MedLab ME in January
In February, LGC Clinical Diagnostics launched the AccuPlex™
SARS-CoV-2 Omicron Variant Reference Material Kit, offering complete SARS-CoV-2 genome coverage with a focus on representative S- and N-gene mutations. This expansion enhances the quality and specificity of our SARS-CoV-2 offering, furthering LGC’s contribution to global disease control efforts.
In March, the VALIDATE® THY2 linearity and calibration verification product was launched with the analyte Thyroglobulin (Tg) for the Beckman Coulter Unicel® DxI/Access instrument. VALIDATE® ISx followed shortly thereafter, including analytes Cyclosporine (CSA)*, Tacrolimus (TAC), and Sirolimus (SIRO) and expanding the VALIDATE offering to over 165 analytes.
An expert panel which took place on March 3rd focused on the promises and challenges of HRD measurement for disease management, including Yves Konigshofer and Russell Garlick. HRD (homologous recombination DNA repair deficiency) creates a permanent genomic scar in cancer cells for many tumor types and is a response biomarker for poly (ADP-ribose) polymerase inhibitor (PARPi) therapy. HRD measurement has the potential to improve cancer therapy, but HRD quantitation remains complex and not broadly available to test providers. The webinar is available to view here.
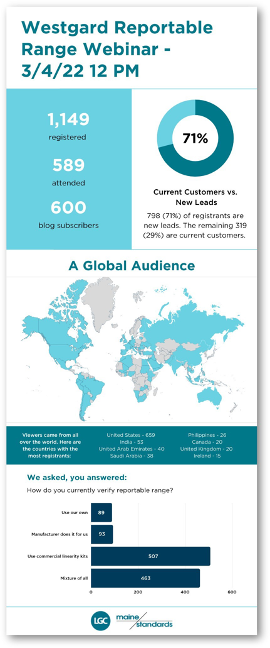
On March 4th, Sten Westgard from Westgard GC hosted a webinar entitled “Toeing the Line: The Right way to verify your Reportable Range and Linearity” that generated plenty of new leads for Clinical Diagnostics. A continuing education credit was available to participants who learned how to collect data for a reportable range study, calculate statistics to determine linearity, graph data for easy visual interpretation, and benchmark the reportable range against performance specifications. That webinar is available to view here. See below for a graphical summary of the results.
Finally, there is a new document search tool available on the Maine Standards website to make it easier for customers to search for inserts and safety data sheets. Site visitors now have the option of using keywords to find their desired inserts or entering the product/lot information directly, improving usability and enhancing the customer experience.

.jpeg)

