Calibration Verification with Linearity Experiements - When Should Non-Linearity Affect a Medical Decision?
Clinical laboratories provide healthcare professionals with vital information to aid in the diagnosis, treatment, and management of patient care. The performance of the assays and analyzers used to carry out these diagnostic tests must therefore be regularly verified by calibration verification (CalVer) to ensure consistently accurate and reliable results.
CalVer ensures that an assay performs properly across the full reportable range – the analytical measurement range (AMR) – as claimed by the manufacturer. This is often achieved by performing linearity experiments using commercially available CalVer/linearity kits – such as LGC Clinical Diagnostics’ VALIDATE® range of liquid, ready-to-use kits – to check for a straight line relationship between the sample analyte concentration and output signal over the AMR. These kits can serve as excellent quality assurance material, but laboratories often lack clear guidance on how to deal with a non-linear response, and whether such results have any clinical relevance.
CalVer regulations
The 1988 Clinical Laboratory Improvement Amendments (CLIA) regulations require laboratories in the United States to verify the performance characteristics of all quantitative test systems, and this is also required for accreditation by the College of American Pathologists (CAP).1,2 A laboratory regulated by CLIA and/or accredited by CAP is therefore required to perform CalVer according to the manufacturer’s schedule and instructions – at least once every six months – as well as after specific events, such as major maintenance, replacement of critical instrument parts, or relocation.
Knowing your limits
Laboratories must establish their own non-linearity limits for the reportable range of each analyte being tested. These should consider the clinical significance of results and, ideally, be independent of errors due to bias or imprecision. CLIA includes published limits for total allowable error – an analytical quality requirement that sets a limit for both the imprecision and bias that are tolerable in a single measurement – for multiple analytes. These limits include all sources of error and can be used as cut-offs for laboratories to set acceptance criteria. Commercial CalVer/Linearity data reduction formats typically use 25-50 % of the total allowable error for allowable deviation from linearity (ADL) limits, or boundaries can be created using coefficient of variation (CV) percentages.
Clinically significant or ‘reasonably close’?
Facilities need to consider what magnitude of deviation from linearity will impact a medical decision when establishing non-linearity limits. Interpretation of non-linear results should always include a consideration from the clinical use perspective for each analyte. In some cases, the degree of non-linearity may be deemed acceptable, with the ‘reasonably close’ results accepted, and the rationale documented for an external reviewer.3 If the degree of non-linearity is deemed unacceptable, then troubleshooting is required to identify any issues with the instrument or reagents if results fall outside of the ADLs.
When peers come in handy
When a non-linear result is encountered, laboratories first need to rule out other causative factors – such as biological bias – before deciding if the issue is potentially clinically significant. Competitive immunoassays and dye-based chemistry assays may also demonstrate non-linearity if the linearity material used for CalVer is manufactured via the ‘equal delta’ method.
Peer group comparison – such as that provided when laboratories submit results from linearity testing on VALIDATE kits to LGC Clinical Diagnostics – can serve as effective justification in these cases. This allows the comparison of linearity results with colleagues using similar methodologies within the same peer group, clustered first by the kit, instruments and analyte, and further grouped in terms of reagents and units of measurement. This type of comparison is helpful to assess the accuracy of the instrument in use and to guide decision-making and troubleshooting. However, if peer group comparison highlights that there is an issue, and troubleshooting cannot clear the problem, then CalVer/Linearity results must be considered invalid, and the laboratory should reach out to the analyzer support team for further investigation.
Conclusion
The purpose of CalVer is to help laboratories ensure that they provide reliable, accurate, and representative results and that patients receive optimal treatment every time. Instead of looking at CalVer/Linearity reports in terms of numbers, careful interpretation is required to determine when a non-linear result may affect medical decision-making.
Commercial linearity kits serve as a practical and effective quality assurance material to demonstrate linearity over the full AMR for each analyte being tested. The VALIDATE range of liquid, ready-to-use linearity kits assists in the performance and documentation of CalVer and linearity testing, and is supplied with MSDRx® data reduction software at no charge for real-time data analysis. Each kit is designed to be instrument specific – with kits available for most major IVD instrument platforms – and the multiple configurations offered can provide a diverse set of ranges that can provide a ‘best fit’ for many other widely used instruments. (Note: LGC Clinical Diagnostics cannot guarantee results for ‘best fit’ applications.)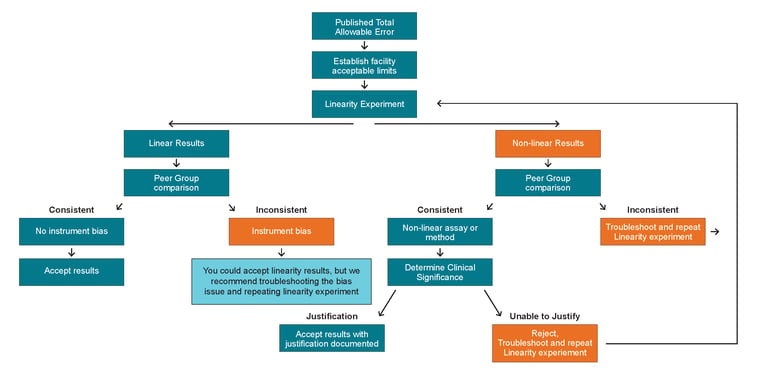 Figure 1. Decision tree for the investigation of non-linearity results in a linearity study, highlighting when a laboratory needs to examine a result for clinical significance.
Figure 1. Decision tree for the investigation of non-linearity results in a linearity study, highlighting when a laboratory needs to examine a result for clinical significance.
| Term | Description |
| Reportable Range | Defined by CLIA as the span of test results over which a laboratory can verify the accuracy of the instrument.2 |
| AMR | Defined by CAP as the range of analyte values that a method can measure directly on a specimen without any dilution, concentration, or other pre-treatment not part of the usual assay process.4 The AMR should not exceed the linear range when comparing measured and true analyte concentrations. |
| CalVer | Defined by CLIA as the process of testing materials of a known concentration as patient samples to ensure that the test system is accurately measuring samples through the reportable range.5 Defined by AP as the process of confirming that the current calibration settings for each analyte remain valid for a period.4 |
| Calibration | The process of establishing a correlation between the measurement signal generated by the instrument and the true concentration of the analyte in the sample.5 |
| AMR Verification | The practice of verifying that the assay system will correctly over the concentration or activity of the analyte over the full range.1 |
| Linearity | In the context of CalVer, linearity refers to the relationship between the final analytical result for a measurement and the concentration of the analyte being measured. Linearity experiments can be used for CalVer, but this is not required, and the term ‘linearity’ is not explicitly used by CLIA. |
| Linearity Testing for CalVer |
The AMR of an analytical system can be validated by demonstrating the straight line relationship between true analyte concentrations and measured concentrations. Both CLIA and CAP require AMR verification using matrix-appropriate materials that span the lower limit, mid-point, and upper-limit of the range. |
Table 1. Glossary of common calibration verification terms.
To download the white paper, click on the button below.
References
-
Calibration Verification/Linearity | College of American Pathologists. Accessed November 11, 2022. https://www.cap.org/laboratory-improvement/proficiency-testing/calibration-verification-linearity
-
CLIA Law & Regulation. Accessed November 15, 2022. https://www.cdc.gov/clia/law-regulations.html
-
The Meaning and Application of Total Error - Westgard. Accessed November 15, 2022. https://www.westgard.com/essay111.htm
-
Chemistry and Toxicology Checklist CAP Accreditation Program. doi:10.24.2022
-
Clinical Laboratory Improvement Amendments (CLIA) Calibration and Calibration Verification - Centers for Medicare & Medicaid Services (CMS).



